Inhibition of amyloid fibril formation of β-lactoglobulin by natural and synthetic curcuminoids†
Abstract
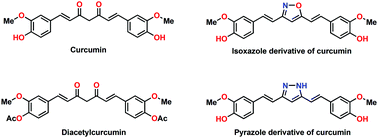
The aggregation of proteins has been associated with several aspects of daily life, including food processing, blood coagulation and many neurodegenerative infections. However, the actual mechanisms responsible for amyloidosis, the irreversible fibril formation of various proteins, which is linked to disorders such as Alzheimer's disease, Creutzfeldt–Jakob disease and Huntington's disease, have not yet been fully elucidated. Curcumin, a potent anti-oxidant, exhibits anti-amyloid activity; however, its activity is limited due to its instability. Therefore, chemical modifications of curcumin have been performed to obtain molecules with enhanced stability and superior anti-amyloid activity. Herein, the main objective of this study is related to the inhibitory effects of three stable analogs of curcumin against bovine β-lactoglobulin (β-lg) fibrillization. We inferred that a pyrazole derivative of curcumin showed remarkable potency in arresting the fibrillization of β-lg, as revealed by biophysical techniques. Molecular docking demonstrated that pyrazole-mediated inhibition of β-lg fibrillogenesis may be initiated by interacting with aggregation-prone regions of the protein and preventing interactions between monomers, leading to suppression of the overall aggregation process. This work alludes to a possible broader scope for discovery of other small molecules that may exert similar effects against amyloid formation and its associated neurodegenerative diseases.


 Please wait while we load your content...
Please wait while we load your content...