Synthesis, absolute configuration and in vitro cytotoxicity of deschloroketamine enantiomers: rediscovered and abused dissociative anaesthetic†
Abstract
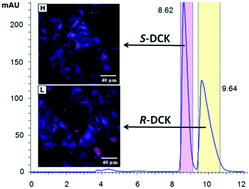
In this study, we aim to determine differences in cytotoxicity of racemic deschloroketamine and its enantiomers. The synthesized racemate of this recently rediscovered and abused dissociative anaesthetic was resolved by chiral HPLC and the absolute configuration of the enantiomers was assigned using a combination of circular dichroism methods and single-crystal X-ray. Not only the absolute configuration, but also the most preferred conformers present in the crystal were successfully determined by electron and vibrational circular dichroism supported by ab initio calculations, and confirmed by X-ray. The in vitro cytotoxicity of racemic deschloroketamine and its enantiomers was determined for nine different types of cell lines. Generally, (S)-deschloroketamine exhibited higher cytotoxicity in the majority of cases. For human embryonic kidney cells (HEK 293T), the (S)-enantiomer reached the IC50 below 1 mM concentration and, in consequence, proved to be twice as potent as the (R)-enantiomer. On the other hand, live-cell fluorescence microscopy imaging at sub-IC50 concentrations provided evidence for only a minor effect of deschloroketamine racemate and enantiomers on endoplasmic reticulum stress and mitochondria morphology in neuroblastoma cells SH-SY5Y.


 Please wait while we load your content...
Please wait while we load your content...