Mesogenic D–A fluorophores based on cyanovinyl and benzothiadiazole†
Abstract
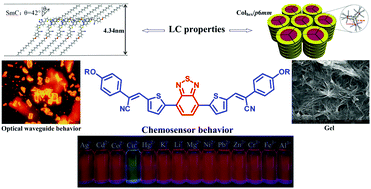
A series of donor–acceptor fluorophores containing the biscyanovinyl dithienebenzothiadiazole central unit with 4-alkoxyphenyl groups at both sides were synthesized. These compounds can display smectic C and hexagonal columnar mesophases in their bulk states, and aggregate into organogels as well as cubic or needle crystals in organic solvents. These cubic or needle crystals exhibit optical waveguide behavior. Additionally, these compounds can exhibit broad absorption up to 625 nm with a narrow band gap of 1.79 eV. The fluorescence emission of these compounds in CHCl3 can be quenched upon addition of C70. Furthermore, these compounds can detect Cu2+ among a series of cations.


 Please wait while we load your content...
Please wait while we load your content...