Heterogeneous oxidative synthesis of quinazolines over OMS-2 under ligand-free conditions†
Abstract
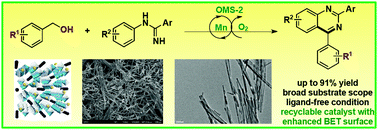
A manganese oxide octahedral molecular sieve (OMS-2) prepared using urea as an additive was found to be an efficient recyclable catalyst for the synthesis of quinazolines via oxidation/cyclization. A broad range of substituted quinazolines were obtained from alcohols and amidines in good yields under ligand-free conditions. OMS-2 was characterized by XRD, BET, ICP-AES, SEM, TEM and XPS, which indicated that the superior catalytic ability might arise from enhanced surface area and crystallinity.


 Please wait while we load your content...
Please wait while we load your content...