Modified hierarchical birnessite-type manganese oxide nanomaterials for CO catalytic oxidation
Abstract
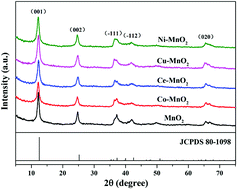
Hierarchical birnessite-type manganese oxide catalysts modified by transition metal (cobalt, cerium, copper, and nickel) cations were synthesized via a facile hydrothermal reaction. The influence of doping elements on the structure and catalytic behavior of the as-prepared catalysts was thoroughly investigated by characterization techniques such as XRD, SEM, TEM, BET, ICP-OES, XPS, FT-IR, TGA, H2-TPR and O2-TPD. The results indicated that the doping of different transition metal cations was found to hardly alter the phase structures and morphologies of the as-obtained birnessite, but displayed distinct differences in catalytic activity for CO oxidation. The doping of Ce(III) and Cu(II) remarkably promoted the catalytic performance while Co(II) and Ni(II) induced a passive effect on the activity. Ce–MnO2 exhibited superior CO oxidation activity at lower temperatures with T50 = 146 °C and T90 = 175 °C in comparison with the pristine birnessite, while Cu–MnO2 possessed the best activity at a relatively low temperature below 120 °C with Tlight-off = 65 °C. The mobility and reducibility of lattice oxygen were found to be the determinants for CO oxidation, in substantial correlation with the generation of surface oxygen vacancies and activation of lattice oxygen species. These features could be mostly ascribed to the formation of synergistic Ce–Mn and Cu–Mn interactions in their respective lattice oxides, however, in different ways.


 Please wait while we load your content...
Please wait while we load your content...