Magnesium/chloride co-doping of lithium vanadium phosphate cathodes for enhanced stable lifetime in lithium-ion batteries†
Abstract
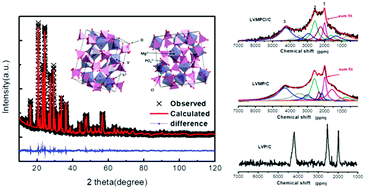
A Mg and Cl co-doped Li3V2(PO4)3/C (LVMPCl/C) material has been synthesized via a solid state method. The effects of Mg and Cl co-doping on the electrochemical properties, structure and morphology of Li3V2(PO4)3 are investigated. Detailed analysis of the XRD patterns suggests that Mg and Cl atoms partly occupy V and O sites in the crystal structure of Li3V2(PO4)3, respectively. The valence states of Mg and Cl elements are investigated using X-ray photoelectron spectroscopy (XPS). Combining XRD patterns with 31P NMR spectra, it is further demonstrated that doped Mg and Cl atoms affect the local electronic structure of P atoms in Li3V2(PO4)3. According to the results of electrochemical performance, LVMPCl/C exhibits excellent discharge capacity as high as 129.1 mA h g−1 at 0.1C. In addition, the capacity retention of LVMPCl/C is almost 100% after 100 cycles at 3.0–4.3 V. Impedance spectroscopy (EIS) and cyclic voltammetry (CV) curves illustrate the lower charge transfer resistance and much more decreased polarization of LVMPCl/C than the pristine one. The excellent electrochemical performance of LVMPCl/C can be attributed to its larger Li ion diffusion channels, which is ascribed to the increased unit-cell volumes, smaller particle sizes and higher electronic conductivity.


 Please wait while we load your content...
Please wait while we load your content...