Host–guest complexation of ionic liquid with α- and β-cyclodextrins: a comparative study by 1H-NMR, 13C-NMR and COSY
Abstract
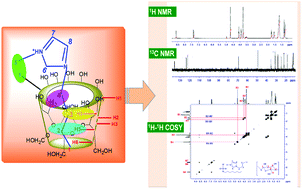
The formation of host–guest inclusion complexes (ICs) by an ionic liquid (IL) 1-butyl-3-methylimidazolium octylsulphate [Bmim][OS] within α- and β-cyclodextrins (CDs) has been explored by 1H NMR, 13C NMR and 2D 1H–1H COSY methods. The mechanism of the CD–[Bmim][OS] host:guest complex formation is systematically presented. The structural effect and various types of interactions, such as, H-bonding, electrostatic force and hydrophobic interactions that are responsible for the inclusion of the IL [Bmim][OS] into the cavity of CDs have been critically discussed with the help of NMR methods. It is observed that the hydrophobic effect plays a crucial role in supporting the formation of CD–[Bmim][OS] ICs. Furthermore, the spectral results obtained from 2D 1H–1H COSY NMR suggest that both H-bonding and electrostatic interactions also make a significant contribution to the stability of the IL–CD ICs.


 Please wait while we load your content...
Please wait while we load your content...