Synthesis, surface properties and temperature dependence of phase separation of DSPE chains in ethanol solutions†
Abstract
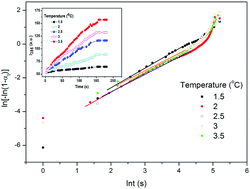
A novel renewable dehydroabietic acid trimethylolpropane ester (DATE) and a dehydroabietic-based succinic acid polyester (DSPE) were successfully prepared in a facile way. Various analytical techniques were employed to evaluate the chemical structure of the ester and the polymer. The monomer and the polymer can form stable micelles at low critical micelle concentrations (CMCs), and the CMC values are 0.0026 and 0.0019 mg mL−1 for DATE and DSPE in ethanol, respectively. In addition, the aggregation and dissociation of DSPE chains in ethanol solutions during one cycle of the heating and cooling process were detected. The results indicated that DSPE chains aggregate during cooling. In contrast, during heating, the aggregates dissociated gradually and distinct hysteresis occurred. Moreover, the kinetics of phase separation of the DSPE/ethanol solution was monitored, and the apparent activation energy of phase separation of the DSPE/ethanol solution was estimated by kinetics analysis. The results showed that DSPE with a low CMC and temperature-dependent behavior may have tremendous scope as a drug carrier in the area of controlled drug delivery.


 Please wait while we load your content...
Please wait while we load your content...