An effective electrochemical sensing platform for fluoride ions based on fluorescein isothiocyanate–MWCNT composite†
Abstract
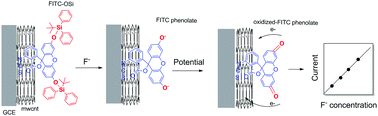
Fluorogenic F− sensors based on fluorescein isothiocyanate (FITC-OSi) are highly selective because they rely on reactions between silica and F−. However, the low intrinsic fluorescence quantum yield of FITC-OSi and the susceptibility of fluorescent signals to be changed by factors other than F− concentration have limited their practical use. Herein, we show that the intrinsic redox properties of FITC-OSi can be used instead of its fluorescence as the transducing signal to overcome these challenges. FITC-OSi and pristine multiwalled carbon nanotubes (p-MWCNTs) were assembled on GCEs to form FITC-OSi–p-MWCNT–GCE electrodes. Cyclic voltammetry was used to analyse its current response in the presence of F− using an experimental design that mimicked the operation of a screen-printed electrode. The electrode generates two anodic current signals at different potentials with F− addition due to the oxidation of FITC phenolates formed during the reactions of silica and F−. This reaction is irreversible and diffusion controlled. The anodic current is proportional to the amount of F− in solution and a maximum RSD of 5% was recorded after repeated experiments. This showed the stability of the electrode and reproducibility of its current response. p-MWCNTs were used to tune the charge transfer properties of the electrode and allow the anodic current to be detected. The method used to incorporate p-MWCNTs into the electrode and their loading significantly affected the current response of the electrode. The electrode worked well in tap water and showed high selectivity, sensitivity, and a low detection limit of 0.26 μM, which was lower than the drinking water standard set by the EPA and WHO. This work shows that reliable and robust F− sensing electrodes based on fluorogenic sensors can be prepared, which opens the door for applications in new technologies, such as screen-printed electrodes.


 Please wait while we load your content...
Please wait while we load your content...