Synthesis and characterization of novel dimeric s-triazine derivatives as potential anti-bacterial agents against MDR clinical isolates†
Abstract
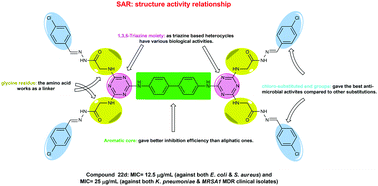
A new library of dimeric s-triazine hydrazide derivatives was synthesized and fully characterized using IR, NMR, and elemental analysis. The synthesized compounds were screened for their in vitro antimicrobial activity. Some of these compounds were found to have more potent anti-bacterial activity than the standard drug ampicillin trihydrate. The compounds which showed the lowest MIC values, such as compounds 1, 12, 13, 20d, 21d and 22d, were further screened for their antibacterial activity against MDR (multi drug resistant) clinical isolates. In contrast to the reference drug ampicillin, the tested compounds revealed significantly low MIC values towards the tested MDR strains. The most active compounds 1, 12, 13, 20d, 21d and 22d showed a high selectivity index towards antimicrobial activity against K. pneumoniae and MRSA1 compared to mammalian cells, suggesting a good safety profile.


 Please wait while we load your content...
Please wait while we load your content...