Solar light active plasmonic Au@TiO2 nanocomposite with superior photocatalytic performance for H2 production and pollutant degradation†
Abstract
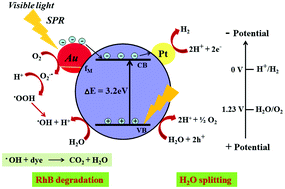
Spherically shaped plasmonic Au nanoparticles (NPs) of size 10 nm (±4 nm) have been decorated on TiO2 NPs for the synthesis of Au@TiO2 composites via an aqueous sol–gel method. The effects of the Au concentration on the optical properties, structural surface properties and photocatalytic activity have been investigated. The optical study indicated characteristics of the surface plasmon resonance (SPR) of Au NPs, which greatly red shifted the photo-absorption from UV to visible light. A Tauc plot showed a downward shift in the band gap energy due to the creation of a metal–semiconductor Schottky junction at the nano Au@TiO2 surface. The well crystallized mixed (anatase and rutile) TiO2 phase formation was investigated using X-ray diffraction studies. The decrease in the photoluminescence (PL) intensity with Au loading indicated an increase in the charge carrier separation. The porous nature of the synthesized photocatalyst material was observed in both Field Emission Scanning Electron Microscopy (FESEM) and Field Emission Transmission Electron Microscopy (FETEM). The homogeneous distribution of Au NPs over the entire TiO2 surface was examined using FETEM and was also confirmed by elemental mapping with STEM. The broadening and shifting of the Raman peak from 143.2 cm−1 to 144.7 cm−1 indicated the generation of crystalline defects such as vacancies and interstitial rearrangement, which may act as trapping sites for electrons. BET surface measurements revealed that the surface area increased from 29.19 m2 g−1 for bare TiO2 to 60.05 m2 g−1 for 2% (w/w) Au loading on TiO2. The synthesized porous Au@TiO2 composites exhibited a high photocatalytic activity for the splitting of H2O under natural solar radiation with the H2 generation rate of 399 μmol/0.1 g h−1, which is much higher than 132 μmol/0.1 g h−1 demonstrated by pristine TiO2 NPs. Along with the hydrogen (H2) generation via water splitting, photocatalytic Rhodamine-B degradation and the kinetics of the reaction were investigated in solar light. The rate was observed to be due to a pseudo-first order reaction.


 Please wait while we load your content...
Please wait while we load your content...