A pH-responsive and magnetic Fe3O4@silica@MIL-100(Fe)/β-CD nanocomposite as a drug nanocarrier: loading and release study of cephalexin†
Abstract
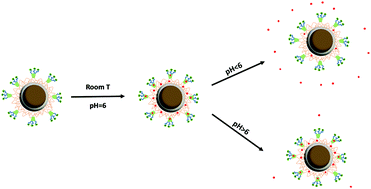
In the present work, a novel magnetic and pH-responsive porous nanocomposite was prepared by the surface grafting of β-cyclodextrin onto Fe3O4@silica@MIL-100(Fe). The effect of pH and temperature on the adsorption of cephalexin and release behavior of the developed nanocomposite was studied. It was found that the maximum adsorption occurs at room temperature while fast release occurs at higher temperature. Among different two-parameter isotherm models, the results showed that the adsorption of the drug was well described by the Langmuir isotherm model. Different kinetic models including film-transfer, intra-particle diffusion, and pseudo-second order kinetic models were used to investigate the mechanism of adsorption. Thermodynamic studies indicated that the adsorption of cephalexin onto the nanocomposite was exothermic and spontaneous. In addition, the nanocomposite showed sensitive pH-dependent behavior. Finally, mathematical kinetic models such as zero-order, first-order, Korsmeyer-Peppas and Higuchi were studied to investigate the drug release profile where the kinetics of release were well described by the Higuchi model.


 Please wait while we load your content...
Please wait while we load your content...