Cinchona derivatives as sustainable and recyclable homogeneous organocatalysts for aza-Markovnikov addition†
Abstract
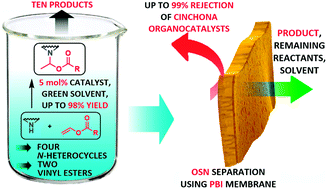
Three cinchona derivatives have shown remarkable activity to catalyze the aza-Markovnikov addition reaction of N-heterocycles to vinyl esters. The synthesis of aza-Markovnikov adducts possessing valuable biological activity was thoroughly optimized. By studying the ratio of the starting materials, bases and solvents, we achieved a new and efficient protocol, which could be performed under mild conditions with a small excess of vinyl ester affording products with excellent yields and high regioselectivity. This optimization reduced Sheldon's E-factor of the reaction by 42%. Furthermore, membrane separation for catalyst recycling was assessed to further improve the sustainability of the synthesis.


 Please wait while we load your content...
Please wait while we load your content...