Synthesis, photophysical, antibacterial and larvicidal studies on triazolophanes with 5-nitro isophthalate functionality at the intraannular position†
Abstract
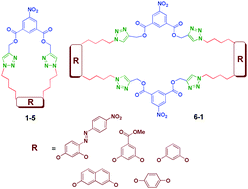
1 : 1 and 2 : 2 oligomeric triazolophanes with 5-nitro isophthalate and triazolyl functionalities at the intraannular position have been synthesized by copper(I) catalyzed 1,3 dipolar cycloaddition of the corresponding propargylic esters with various phenyl substituted aliphatic azides under mild conditions through click methodology. The 1 : 1 oligomeric triazolophanes exhibit good target binding ability and better antibacterial activity against Staphylococcus aureus, Bacillus subtilis, Salmonella typhi and Escherichia coli bacteria than the 2 : 2 oligomeric triazolophanes, supported by molecular docking studies, and triazolophanes T3, T4 and T5 show good larvicidal activity.


 Please wait while we load your content...
Please wait while we load your content...