Ligand and electronic effects on copper–arylnitroso self-assembly†
Abstract
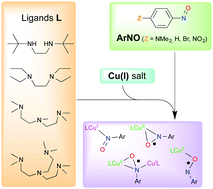
A series of complexes were prepared by self-assembly of copper(I) precursors and arylnitroso species. The nature of the copper(I) supporting ligand (bi-, tri- or tetradentate as well as secondary vs. tertiary amine donors) and the electronic nature of the arylnitroso species (electron-donating or withdrawing substituents) were varied. The stoichiometry of the reaction, the topology and the electronic properties of the adducts were characterized by means of UV-vis spectroscopy, X-ray diffraction and DFT methods. The more electron-rich ligands and the more electron-poor arylnitroso species lead to an inner-sphere electron-transfer and formation of copper(II)–(arylnitrosyl radical) complexes, with a linkage topology that depends on the denticity of the supporting ligand. These results provide a canvas by which the products of similar self-assembled redox reactions can be predicted.
- This article is part of the themed collection: Equilibrium Solution Coordination Chemistry


 Please wait while we load your content...
Please wait while we load your content...