Nitrogen reduction utilizing solvated electrons produced by thermal excitation of trapped electrons in reduced titanium oxide
Abstract
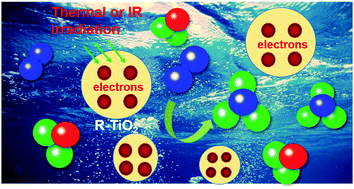
Heterogeneous catalytic dinitrogen reduction to produce ammonia at ambient pressure and temperature is a challenging task when attempting to mimic the natural dinitrogen fixation process because the N2 molecule hardly binds with solid catalytic materials and the generation of high energy intermediates is required. Energetic solvated electrons, as highly reactive species, can directly react in solution and thus can overcome such limitations in ammonia synthesis. Although solvated electrons are commonly generated by high energy radiation, a facile approach for generation of solvated electrons is of increasing importance. Previous literature reported that photo-illuminated hydrogen-terminated diamond yields solvated electrons for reduction of N2. In this study, the solvated electrons were produced by direct emission of stabilized electrons from heavily reduced TiO2 into water under thermal or infrared light excitation. The structural change of the reduced TiO2 was characterized to understand the process of stabilization and release of excess electrons. Furthermore, the conversion of N2 into NH3 at different conditions under thermal excitation was tested to evaluate the reduction activity of solvated electrons. The thermal energy-controlled electron emission from the reduced TiO2 particles into solution has great application potentials in areas of energy conversion, chemical synthesis, and medical treatment.


 Please wait while we load your content...
Please wait while we load your content...