Theoretical study on the effective dehydrochlorination of 1,2-dichloroethane catalyzed by tetraalkylphosphonium chlorides: electrostatically controlled reactivity†
Abstract
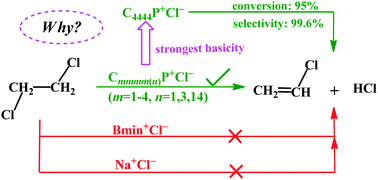
This work aims at understanding the intriguing experimental observation of the most effective dehydrochlorination of 1,2-dichloroethane (DCE) catalyzed by tetrabutylphosphonium chloride (C4444P+Cl−) vs. other tetraalkylphosphonium chlorides, imidazolium chlorides, and inorganic molten salts. The calculation on the C4444P+Cl−-catalyzed DCE dehydrochlorination reaction shows a bimolecular elimination (E2) mechanism, where the ionic liquid (IL) plays an amphiphilic dual activation role, whose component anion and cation act cooperatively to induce the cleavage of the C–H and C–Cl bonds of DCE and the formation of the H–Cl bond. The effective dehydrochlorination of DCE catalyzed by C4444P+Cl− IL is attributed to the strong basicity of the IL, which originates from the weak electrostatic interaction between the anionic and cationic partners in comparison with other tetraalkylphosphonium chlorides, imidazolium chlorides, and inorganic molten salts. The theoretical calculations emphasize the electrostatically controlled character of DCE dehydrochlorination in the ILs as well as the cooperative catalysis of ILs. The present results would provide guidance for understanding the reactivity of the IL-catalyzed dehydrochlorination of DCE.


 Please wait while we load your content...
Please wait while we load your content...