A Cu(ii) mediated approach for colorimetric detection of aqueous fluoride in ppm level with a Schiff base receptor†
Abstract
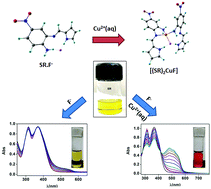
Inspired by the importance of fluoride detection in aqueous environment, a new methodology is demonstrated by employing the synergistic reaction of fluoride and Cu(II) salts with a designed Schiff base receptor. The cooperative ion binding behaviour of the ligand SR involves the reduction of Cu(II) centre to Cu(I) in the presence of fluoride. In chloroform solution, the sensor shows equivalent reversible colorimetric change from amber yellow to red irrespective of the order of the addition of copper and fluoride. However, the change is equivalent but reversibility cannot not be attained in DMSO. The ion binding event can be easily recycled by adding EDTA and the resulting reversible colorimetric change mimics AND and a combinatorial logic gate. The mechanism of interaction is thoroughly analyzed by the UV-Vis, 1H NMR, 19F NMR, ESR spectroscopy and cyclic voltammetry techniques, which is further supported by DFT calculations. The methodology is applied in the detection of fluoride in real life samples such as ground water and toothpaste extract. The method successfully detects fluoride at concentrations as low as 5 ppm.


 Please wait while we load your content...
Please wait while we load your content...