Synthesis and characterization of cyano and isocyano complexes of bis(dithiolato) molybdenum using Me3SiCN: a route to a cyanide-bridged multimer to a monomer†
Abstract
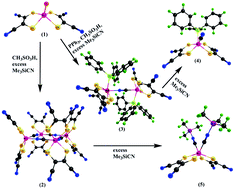
Cyanide- and isocyanide-bound molybdenum complexes containing maleonitriledithiolate (mnt = 1,2-dicyanoethylenedithiolate = S2C2(CN)2) as coligands were prepared from the synthon [Et4N]2[MoIVO(mnt)2] (1) as [Et4N][(PPh3)(mnt)23−˙MoIII(μ-CN)MoIII(PPh3)(mnt)23−˙] (3), [Et4N][MoIII(PPh3)(CN)(mnt)23−˙]·CH2Cl2 (4), and [MoIII(CNSiMe3)2(mnt)23−˙](5) using trimethylsilylcyanide (Me3SiCN). Triphenylphosphine (PPh3) was used as a blocking group and also as a competing ligand to cyanide to control the geometry and reactivity for forming the cyanide-bridged molybdenum dimer 3 or with terminal cyanide for the monomer 4. The known tetramer [Et4N]4[MoIII4(μ-CN)4(mnt)8] (2) formed in the absence of PPh3 showed a remarkable reaction with the excess Me3SiCN, where the monomeric species [MoIII(CNSiMe3)2(mnt)2] (5) was formed with Me3SiNC coordination. Overall, the Mo(IV) bis (dithiolate) complex showed diverse reactions with Me3SiCN in the presence and absence of the coligand PPh3 and under protic and aprotic media. The precursor molybdenum complex in the IV oxidation state was reduced to the III state in the complexes 3, 4, and 5, where one of the coordinating chelating ligand mnt2− was oxidized to mnt1−˙. This chemistry was supported by the EPR, electrochemical studies and X-ray crystallography results and corroborated by the DFT level of calculations. The complexes 3 and 4 were blue-emitting materials with a long lifetime in the millisecond range. Further DFT and TD-DFT calculations were carried out to understand the electronic states and the origin of the electronic absorptions.


 Please wait while we load your content...
Please wait while we load your content...