Au–Fe nanoparticles visited by MD simulation: structural and thermodynamic properties affected by chemical composition†
Abstract
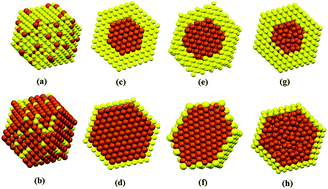
In this work, Fe–Au nanoalloys and Fe@Au core–shell nanoclusters are investigated via classical molecular dynamics simulation to determine the effect of their composition on their thermodynamic stability and melting mechanism. Accordingly, several analysis methods are used including potential energy, heat capacity, common neighbor analysis, structural stability parameter, displacement vector, volumetric strain, volumetric displacement, and order parameter. For the Fe@Au core–shell nanoclusters, three different core structures including fcc-core, bcc-core, and amorphous-core are considered. The results indicate that nanoclusters with a higher Fe concentration exhibit greater thermodynamic stability. Also, for all the studied compositions, the Fe–Au nanoalloy demonstrates the highest thermodynamic stability and melting point. Moreover, the melting mechanism of all the simulated nanoclusters is found to be the same, which is independent of the nanocluster composition and structure. For all the studied nanoclusters, melting starts from the shell region then extends to the core until the nanocluster is totally melted. Several solid–solid transitions are observed before melting, which are accompanied with variations in the fcc, hcp, and other local ordered structures. Moreover, the simulations indicate that the atoms on the faces of the nanoclusters exhibit greater displacement and strain in comparison with that on the vertices and edges.


 Please wait while we load your content...
Please wait while we load your content...