A sensitive and selective sensor for picric acid detection with a fluorescence switching response†
Abstract
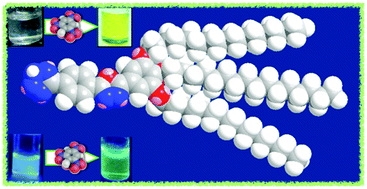
A low molecular weight organogelator containing 3,5-substituted-1,3,4-oxadiazole and tetrazole units was synthesized and characterized. This compound is only soluble in DMSO and forms a stable gel. The solution and gel exhibit a blue light emission. The gel was characterized by atomic force microscopy, field-emission scanning electron microscopy, 1H NMR and fluorescence measurements. The gel to solution interconversion was reversible for many cycles of heating and cooling. The compound in solution exhibited a high selectivity for the detection of picric acid, a common explosive and water pollutant. Fluorimetric titration studies with nitro explosive compounds revealed that the emission of the compound was red shifted in response to the addition of picric acid, and exhibited a shifting of fluorescence from blue to green. Theoretical and experimental studies revealed that the sensing is due to the complexation of the picrate anion with the protonated fluorophore. The shifting of emission in response to picric acid in the visible region is ideal for the naked eye detection of explosives and therefore it is promising in comparison to the detection methods based on fluorescence quenching.


 Please wait while we load your content...
Please wait while we load your content...