Spontaneous disproportionation of lithium biphenyl in solution: a combined experimental and theoretical study†
Abstract
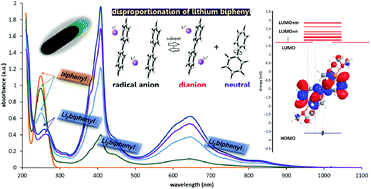
In the present paper we report experimental and theoretical evidence for lithium biphenyl disproportionation in solution. The presence of an absorption band (at 250 nm), which corresponds to neutral biphenyl in the spectra of dissolved crystalline [Li+(THP)4][Bph˙−] (1), clearly suggests the disproportionation of the biphenyl radical anion into neutral biphenyl plus the corresponding dianion, 2Bph˙− ⇄ Bph2− + Bph0. The experimental spectrum of 1 displays four main groups of bands at 834, 644, 408 and 250 nm. Upon addition of an excess of lithium, the biphenyl band becomes diminished revealing a hidden lower intensity band at 262 nm, this one truly belonging to the dianion. Highly accurate time-dependent density functional theory (TDDFT) calculations of the electronic spectra of a series of contact as well as solvent separated ionic associate models, performed at the wB97XD/6-311++G(3df,3pd) level of theory in dimethoxyethane (DME) solution, revealed that the contact lithium biphenyl dianion [(Li+DME)2Bph2−] predicts all the main absorption bands fairly well, while the corresponding radical anion does not reproduce the experimental spectral pattern. Analysis of the electron density distribution performed by means of Quantum Theory of Atoms in Molecules (QTAIM) confirms that the studied ionic associates represent the correct resonance structures since the charges of the lithium cations are close to +1 in all the cases. Solvation usually plays a key role in these types of equilibria; however the underlying cause of this disproportionation seems to lie on the inherent electronic stabilities of the anionic species involved, as could be inferred from the reported free energy calculations of the nude ion associates, neglecting any solvent effects.


 Please wait while we load your content...
Please wait while we load your content...