New amide based iridium(iii) complexes: synthesis, characterization, photoluminescence and DFT/TD-DFT studies†
Abstract
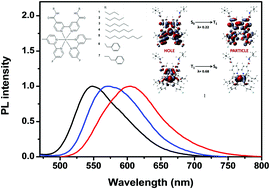
New iridium(III) complexes with 2-(3-fluorophenyl)-4-methylpyridine as cyclometalated ligands and 2,2′-bipyridine-4,4′-dicarboxamide derivatives as ancillary ligands were synthesized and characterized by FTIR, NMR, MS, UV/Visible, and fluorescence spectroscopies and cyclic voltammetry. The effects of different solvents and substituents on the photophysical properties of the complexes have been investigated. The complexes show tunable photoluminescence wavelengths depending on solvent polarity in the 548 nm to 610 nm range where the emission colour can change from green to orange-red. The complexes exhibit high photoluminescence quantum yields between 0.41 and 0.75, which may be attributed to the change in the transition dipole moment originating from the amide group upon electronic excitation. The calculated HOMO and LUMO energy levels of complexes 1–7 are in the −5.51 to −5.60 eV and −3.37 to −3.40 eV ranges, respectively. The ground and excited state properties of 1 have been fully investigated by means DFT and TD-DFT methods respectively. The general trends of observed data have been successfully represented and quantified by computational data.


 Please wait while we load your content...
Please wait while we load your content...