Ultrasound-assisted one-pot multicomponent 1,3-dipolar cycloaddition strategy: combinatorial synthesis of spiro-acenaphthylene-S,S-acetal and 2H-pyranone derivatives†
Abstract
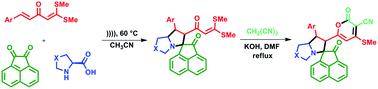
The stereo/regio/chemoselective syntheses of a library of novel spiro[acenaphthylene-1,3′-pyrrolizin]-2-one and spiro[acenaphthylene-1,5′-pyrrolo[1,2-c]thiazol]-2-one have been achieved through 1,3-dipolar cycloaddition. Intermediate azomethine ylides generated in situ from α-amino acids and acenaphthylene-1,2-dione react with α-aroylidine ketenedithioacetal (AKDTA) derivatives to give the target product in excellent yield under ultrasound irradiation. Further, spiro-S,S-acetals are converted to spiro-2H-pyranones by a one-pot cyclization strategy.


 Please wait while we load your content...
Please wait while we load your content...