A highly selective and sensitive chemosensor for l-tryptophan by employing a Schiff based Cu(ii) complex†
Abstract
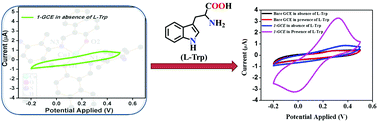
A new Cu(II) complex C40H38N4CuO2 (1) was synthesized and characterized by elemental analysis, mass spectrometry and also authenticated by single crystal X-ray studies. Structural analysis of 1 reveals the distorted square planar geometry around the Cu(II) centre and the presence of C–H⋯O and N–H⋯π hydrogen bonding interactions. The sensing behaviour of 1 towards L-tryptophan (L-Trp) was identified by UV-vis spectroscopic and cyclic voltammetry experiments. In the UV titration of 1 with L-Trp, four distinct isosbestic points were observed. Furthermore, on modifying the surface of the glassy carbon electrode (GCE) by 1 (1-GCE) the oxidation potential of L-Trp at +0.32 V is revealed, which is considerably lower than the values previously reported for other electrodes. Additionally, the 1-GCE can detect L-Trp with a noteworthy detection limit (185 nM), sensitivity (3.156 μA μM−1 cm−2) and with distinguished selectivity in the presence of various other amino acids. The 1-GCE also displays excellent reproducibility, repeatability and long term stability. Finally, electrochemical impedance spectroscopy (EIS) investigations were carried out to gauge the effects of modification with 1 on the characteristics of the GCE. Moreover, the molecular docking study of 1 with various proteins reveals the π–π stacking interaction with L-Trp.


 Please wait while we load your content...
Please wait while we load your content...