Reactions of a series of ZnL, CuL and NiL Schiff base and non-Schiff base complexes with MCl2 salts (M = Cu, Ni, Mn): syntheses, structures, magnetic properties and DFT calculations†
Abstract
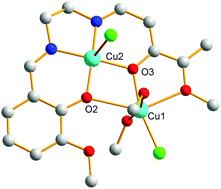
We report the syntheses, structural determination and magnetic properties of a few 3d Schiff base metal complexes resulting from the reaction of preformed 3d Schiff base metal complexes with MCl2 salts (M = Cu, Ni, Mn). Different behaviors can occur, from a metal exchange reaction to coordination of M ions and chloride anions or, in the presence of alkaline ions, to formation of tetrachlorocuprate entities that behave as counter-anions in the final structures. So use of o-vanillin-derived Schiff base ligands possessing two coordination sites of different sizes allows isolation of a genuine dicopper complex entity involving a highly non symmetric diphenoxo bridge characterized by a central Cu–O2–Cu core possessing very different Cu–O bond lengths and Cu–O–Cu angles. Use of o-vanillin as a chelating ligand in place of o-vanillin-derived Schiff base ligands furnishes a supplementary degree of freedom in the syntheses of new complexes. The two o-vanillin ligands can rearrange to yield cubane complexes or give heterodinuclear complexes. DFT calculations demonstrate that in the genuine non-symmetric diphenoxo bridge, the two different bridging pathways have to be taken into consideration to explain the resulting weak antiferromagnetic interaction in the dicopper complex, an interaction resulting from non-negligible antagonistic antiferro- and ferromagnetic interactions.


 Please wait while we load your content...
Please wait while we load your content...