Azasilicon-bridged heterocyclic arylamines: syntheses, structures and photophysical properties†
Abstract
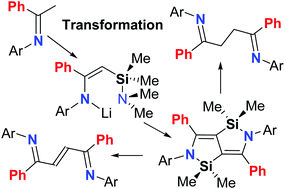
The lithium κ1-enamides, Me2NSiMe2CHC(Ph)-{2,6-(R1)2-4-(R2)C6H2}NLi·3THF (R1 = iPr, R2 = H L1; R1 = Et, R2 = H L2; R1 = Me, R2 = H L3; R1 = R2 = Me L4; R1 = Et, R2 = Me L5), in the presence of titanium tetrachloride, undergo intermolecular rearrangement cyclization reactions resulting in 1,3-migration of the silicon groups and the elimination of dimethylamine affording five examples of bis-azasilicon-bridged heterocyclic arylamines, [{2,6-(R1)2-4-(R2)C6H2}N(Ph)CCSiMe2]2 (R1 = iPr, R2 = H D1; R1 = Et, R2 = H D2; R1 = Me, R2 = H D3; R1 = R2 = Me D4; R1 = Et, R2 = Me D5) in good yield, respectively. The molecular structures of D1–D5 show the two fused N–Si–C–C–C rings to be co-planar indicative of extended π-conjugation, while their photophysical properties reveal them to be green/blue emitting with high luminescence quantum yields (ΦF range: 75–99%). Furthermore, the compounds D serve as versatile reactants undergoing ring opening on hydrolysis to afford the saturated 1,4-diimines [{2,6-(R1)2-4-(R2)C6H2}N(Ph)C-CH2]2 (R1 = iPr, R2 = H E1; R1 = Et, R2 = H E2; R1 = Me, R2 = H E3; R1 = R2 = Me E4; R1 = Et, R2 = Me E5). Alternatively, D can be employed in a redox-promoted cascade reaction to afford the conjugated 1,4-diimines, (E)-[{2,6-(R1)2-4-(R2)C6H2}N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(Ph)CH]2 (R1 = iPr, R2 = H F1; R1 = Et, R2 = H F2; R1 = Me, R2 = H F3; R1 = R2 = Me F4; R1 = Et, R2 = Me F5). In addition to D1–D5, E1–E3, E5, F2 and F3 have been the subject of single crystal X-ray diffraction studies.
C(Ph)CH]2 (R1 = iPr, R2 = H F1; R1 = Et, R2 = H F2; R1 = Me, R2 = H F3; R1 = R2 = Me F4; R1 = Et, R2 = Me F5). In addition to D1–D5, E1–E3, E5, F2 and F3 have been the subject of single crystal X-ray diffraction studies.


 Please wait while we load your content...
Please wait while we load your content...