Contemporary progress in the synthesis and reactions of 2-aminobenzothiazole: a review
Abstract
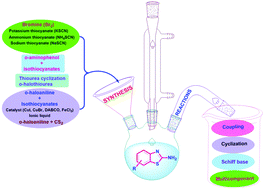
In the past few decades, heterocyclic compounds and their derivatives have gained popularity due to their prominent medicinal importance. The versatile and synthetically accessible 2-aminobenzothiazole scaffolds are quite fascinating for multiple applications in both synthetic organic chemistry and biological fields due to their potent pharmacological activities. A large number of efforts have been made to develop a wide range of methodologies for the synthesis of 2-aminobenzothiazole and its derivatives. Moreover, 2-aminobenzothiazole significantly serves as a reactant or a reaction intermediate for affording various fused heterocycles since NH2 and endocyclic N functions of 2-aminobenzothiazole are suitable to assist reactions with common bis electrophilic reagents to form a diverse fused heterocyclic scaffold. This review highlights various synthetic methodologies and chemical reactions of 2-aminobenzothiazole.


 Please wait while we load your content...
Please wait while we load your content...