The role of aromatic side chains on the supramolecular hydrogelation of naphthalimide/dipeptide conjugates†
Abstract
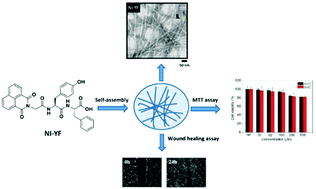
Herein, the influence of an amino acid side chain on the structural and functional properties of hydrogels has been described. In this study, four new hydrogelators based on the conjugates of dipeptides and naphthalimide (NI) have been designed, synthesized, and examined. Homopeptide 1 (NI-FF) of the phenylalanine residue forms a weak hydrogel or a viscous solution of worm-like micelles under basic conditions. On the other hand, dipeptide conjugates of 2 (NI-FY), 3 (NI-YF), and 4 (NI-YY), containing additional hydroxyl groups as tyrosine residues, can self-assemble into supramolecular hydrogels under physiological pH conditions. UV-vis, IR, and rheological studies clearly indicate the formation and stability of these dipeptide hydrogels. The TEM results revealed that the hydrogels (1–4) self-assembled into a nanofibrous morphology. Furthermore, the cell culture results show that NI-YF has better cell compatibility as compared to NI-FY and NI-YY and thus shows potential to be used as a hydrogel biomaterial. Overall, this study highlights the importance of the structure–hydrogelation relationship and provides new insights into the design of self-assembled peptide amphiphiles.


 Please wait while we load your content...
Please wait while we load your content...