A facile atom economic one pot multicomponent synthesis of bioactive spiro-indenoquinoxaline pyrrolizines as potent antioxidants and anti-cancer agents†
Abstract
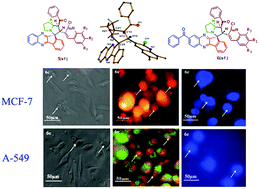
A competent and highly discriminating one-pot method for the synthesis of biologically active novel spiro-indenoquinoxaline pyrrolizines accumulating three pharmocophoric cores, heterocyclic quinoline scaffold, pyrrolizines and indeno-quinoxaline, in a single molecular framework by means of a four-component reaction between ninhydrin, o-phenylenediamine, L-proline and quinolinyl chalcones in methanol via [3+2] cycloaddition was developed. The structures of the compounds were well characterized by FT-IR, NMR, ESI-MS, XRD and elemental analysis. The synthesized compounds were screened for in vitro antioxidant activity using DPPH, nitric oxide, super oxide radical and in vitro cytotoxic activity against MCF-7 and A-549 cancer cell lines and visualized using the fluorescent microscopic technique. The newly synthesized compounds exhibited excellent antioxidant activity compared to the standard molecule, i.e., BHT. In terms of cytotoxic activity, compounds 5c, 5e, 6c and 6e were found to exhibit significant activity, when compared to standard doxorubicin against MCF-7 and A-549 cancer cells using the MTT assay method. The cell morphology analysis clearly indicates cell death was induced by apoptosis and necrosis pathways. Molecular docking studies were performed using an EGFR inhibitor to determine the molecular interactions between the compounds and proteins.


 Please wait while we load your content...
Please wait while we load your content...