Metabolic deregulation in prostate cancer†
Abstract
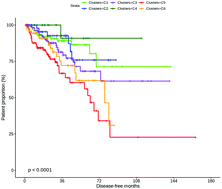
Introduction: The prostate exhibits a unique metabolism that changes during initial neoplasia to aggressive prostate cancer (PCa) and metastasis. The study of PCa metabolism thus represents a new avenue for diagnostics, particularly early diagnosis of aggressive PCa cases. Results: Here, by clustering tissue transcriptomics data from The Cancer Genome Atlas (498 PCa patients), we identified six metabolic subgroups (C1–C6) of PCa that show distinct disease-free survival (DFS) outcomes (p < 0.0001). In particular, we identified at least two subgroups (C5 & C3) that exhibit significant poor prognosis (∼70% and 30–40% relapse within the first 72 months; hazards ratios of 9.4 and 4.4, respectively, relative to the best prognosis cluster C4 that showed <20% relapse even by 120 months). We were able to reproduce the subgroups in several independent datasets including B. S. Taylor et al. (2010) data; 215 patients; DFS p = 0.00088) using a multinomial regression classifier. The subgroups displayed distinct metabolic profiles vis-à-vis normal tissues, measured as ‘deregulation’ observed for 20 metabolic pathways (using Pathifier; Y. Drier and E. Domany, 2013). In particular, C5 and C3 showed considerable deregulation for pathways involved in synthesis and catabolism of complex forms of lipids and carbohydrates, and these were exhibited in parallel or in the face of glycolysis, a common form of energy production in cancer cells. The subgroups were significantly over-enriched for different sets of genetic alterations [BRCA1, MSH2, FOXA1, TP53 (C5), RB1 and STK11(C3); and AR (C1); p ≤ 8.6 × 10−4], suggesting that distinct sets of alterations underpinning the PCa subgroups that ‘push’ the subgroups towards their unique metabolic profiles. Finally, applying the classifier to blood protein expression profiles from 42 active surveillance (AS) and 65 advanced castrate resistant PCa (ACRPC) patients (D. Olmos et al., 2012) assigned 70.77% ACPRC and interestingly reassigned 59.52% AS patients to at least one of the poor prognosis subgroups with 35.71% to the metabolically active poor-prognosis subgroup C3. Conclusion: The identification of PCa subgroups displaying distinct clinical outcomes solely from metabolic expression profiles of PCa tumours reiterates the significant link between deregulated metabolism and PCa outcomes (E. Eidelman et al., 2017). On the other hand, the time to biochemical relapse (rise in PSA levels) was not indicative of early relapse seen for subgroups C3 and C5 (these show considerably late BCR compared to C4). Our study thus highlights specific processes (elevated lipid and carbohydrate metabolism pathways) that could be better indicators than PSA for early diagnosis of aggressive PCa. Availability: https://maxwellplus.com/research/metabolic-deregulation-in-prostate-cancer/.


 Please wait while we load your content...
Please wait while we load your content...