Design and synthesis of conformationally constraint Dyrk1A inhibitors by creating an intramolecular H-bond involving a benzothiazole core†
Abstract
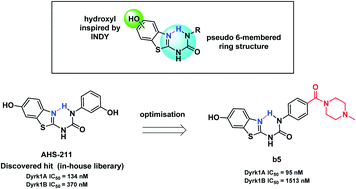
We present the development of conformationally pre-organised Dyrk1A inhibitors based on the hydroxybenzothiazole urea scaffold. The modifications introduced to the discovered hit (AHS-211) proved the crucial role of the urea linker to preserve the bioactive conformation and led to the development of compound b5 as a promising selective Dyrk1A inhibitor.


 Please wait while we load your content...
Please wait while we load your content...