Design, synthesis and bioactivity investigation of tetrandrine derivatives as potential anti-cancer agents†
Abstract
Twenty-four 14-sulfonamide–tetrandrine derivatives as potential anti-cancer agents were synthesized. The synthetic derivatives were investigated for their cytotoxic activity against human cancer cell lines MDA-MB-231, PC3, WM9, HEL and K562. Initially, the IC50 values (50% inhibitory concentrations) of all of the compounds were determined. These derivatives exhibited potent, but distinct, inhibitory effects on the above-mentioned cell lines. Among them, compound 23, which was modified with a 2-naphthalenesulfonyl group at the 14-amino position, showed impressive inhibition of all five cancer cell lines, and especially of MDA-MB-231 cells with an IC50 value of 1.18 ± 0.14 μM. Further mechanism exploration showed that 23 induced potent apoptotic cell death on MDA-MB-231 cancer cells in a concentration-dependent manner. The results revealed that 23 might be a potential anti-cancer drug candidate.
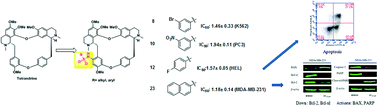


 Please wait while we load your content...
Please wait while we load your content...