Effect of N-1 arylation of monastrol on kinesin Eg5 inhibition in glioma cell lines†
Abstract
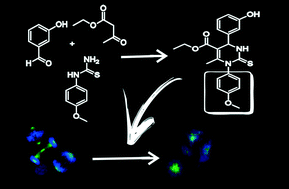
An original and focused library of two sets of dihydropyrimidin-2-thiones (DHPMs) substituted with N-1 aryl groups derived from monastrol was designed and synthesized in order to discover a more effective Eg5 ligand than the template. Based on molecular docking studies, four ligands were selected to perform pharmacological investigations against two glioma cell lines. The results led to the discovery of two original compounds, called 20h and 20e, with an anti-proliferative effects, achieving IC50 values of about half that of the IC50 of monastrol in both cell lines. As with monastrol, flow cytometry analyses showed that the 20e and 20h compounds induced cell cycle arrest in the G2/M phase, and immunocytochemistry essays revealed the formation of monopolar spindles due to Eg5 inhibition without any toxicity to Caenorhabditis elegans.


 Please wait while we load your content...
Please wait while we load your content...