Isolation, expression and biochemical characterization of recombinant hyoscyamine-6β-hydroxylase from Brugmansia sanguinea – tuning the scopolamine production†
Abstract
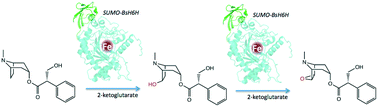
Hyoscyamine-6β-hydroxylase (H6H, EC 1.14.11.11) is a plant enzyme that catalyses the last two steps in the biosynthesis of the anticholinergic drug scopolamine, i.e. the hydroxylation of hyoscyamine to 6β-hydroxyhyoscyamine (anisodamine) and subsequent oxidative ring-closure to the 6,7-β-epoxide. A H6H gene homologue was isolated from the plant Brugmansia sanguinea (BsH6H) and recombinantly cloned into Escherichia coli, expressed and purified using an effective SUMO-fusion procedure. Enzymatic activity is approximately 40-fold higher for the first reaction step and the substrate affinity is comparable to other characterized H6H homologues (Km ∼ 60 μM). Truncation of an H6H enzyme flexible N-terminal region yields an active and stable yet more compact enzyme version.


 Please wait while we load your content...
Please wait while we load your content...