Design, synthesis and antimycobacterial activity of hybrid molecules combining pyrazinamide with a 4-phenylthiazol-2-amine scaffold†
Abstract
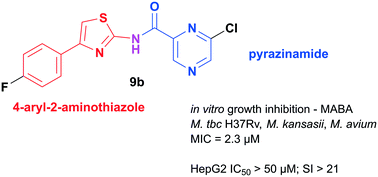
Hybrid compounds based on a combination of the first-line antitubercular pyrazinamide (PZA) and a formerly identified antimycobacterial scaffold of 4-arylthiazol-2-amine were designed. Eighteen compounds were prepared, characterized and tested for in vitro growth inhibition activity against M. tuberculosis H37Rv, M. kansasii, M. avium and M. smegmatis by Microplate Alamar Blue Assay at neutral pH. Active compounds were tested for in vitro cytotoxicity in the human hepatocellular carcinoma cell line (HepG2). The most active 6-chloro-N-[4-(4-fluorophenyl)thiazol-2-yl]pyrazine-2-carboxamide (9b) also had the broadest spectrum of activity and inhibited M. tuberculosis, M. kansasii, and M. avium with MIC = 0.78 μg mL−1 (2.3 μM) and a selectivity index related to HepG2 cells of SI > 20. Structure–activity relationships within the series are discussed. Based on its structural similarity to known inhibitors and the results of a molecular docking study, we suggest mycobacterial beta-ketoacyl-(acyl-carrier-protein) synthase III (FabH) as a potential target.


 Please wait while we load your content...
Please wait while we load your content...