Teratogen screening with human pluripotent stem cells
Abstract
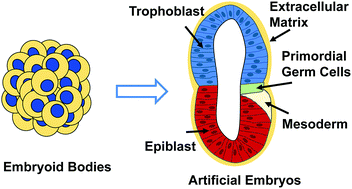
Birth defects are a common occurrence in the United States and worldwide. Currently, evaluation of potential developmental toxicants (i.e., teratogens) relies heavily on animal-based models which do not always adequately mimic human development. In order to address this, researchers are developing in vitro human-based models which utilize human pluripotent stem cells (hPSCs) to assess the teratogenic potential of chemical substances. The field of human developmental toxicity assays includes a variety of platforms including monolayer, micropattern, embryoid body, and 3D organoid cultures. In this review, we will overview the field of human teratogenic assays, detail the most recent advances, and discuss current limitations and future perspectives.


 Please wait while we load your content...
Please wait while we load your content...
