Electrooxidation of sulfate paired to electroreduction of copper for regeneration of persulfate/sulfuric acid etching solution
Abstract
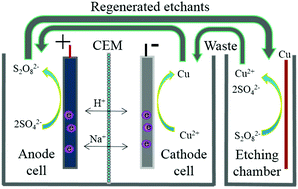
A laboratory scale closed-loop cycle system for the complete regeneration of sodium persulfate/sulfuric acid etching solution (SPS) has been developed by anode oxidation of sulfate paired to cathode reduction of copper. A current-efficient (110%), zero-chemical dosage and zero-emission strategy for complete regeneration of SPS and recovery of copper was achieved. The micro-etching rate of the recycled SPS has no significant difference compared with that of the fresh SPS. On average, the cost of etching in this recycling system is decreased by 100%, compared with that in the current printed circuit boards used in industries. The full utilization of spent SPS realizes waste to wealth conversion in the true sense. The developed recycling system is a techno-economically feasible, environmentally friendly, sustainable, clean and green process for the reuse of SPS and recovery of valuable metal Cu through electrooxidation of sulfate paired to electroreduction of copper.


 Please wait while we load your content...
Please wait while we load your content...