Solvothermal liquefaction of alkali lignin to obtain a high yield of aromatic monomers while suppressing solvent consumption†
Abstract
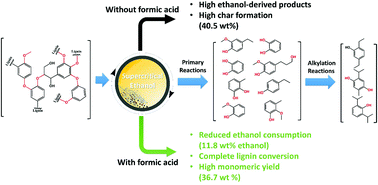
The unique physicochemical properties and high solubility of a wide range of biomass-derived feedstocks make sub- and supercritical alcohols promising media for thermochemical conversion to liquid fuels and value-added chemicals. Short-chain alcohols (C1–C3) not only hydrogenolyse a variety of recalcitrant feedstocks by donating in situ hydrogen, but also suppress the char formation by capping reactive intermediates. However, the beneficial features of supercritical alcohols also bring some demerits, such as their excessive decomposition and high consumption, which has been given cursory attention to date. Consequently, the aim of this study was to elucidate the role of sub- and supercritical alcohols as a hydrogen donor, their self-reactivity, their reactivity with the feedstock, the extent of their conversion under catalytic and non-catalytic conditions, and the detailed pathways to byproduct formation. Based on the solvent reactivity, the optimum conditions were investigated for the solvothermal liquefaction of recalcitrant alkali lignin to give a high yield of aromatic monomers with careful emphasis on the solvent consumption. The addition of formic acid instead of the more commonly used hydrodeoxygenation catalysts (e.g., CoMo/Al2O3, Ru/Al2O3) can not only suppress ethanol consumption significantly (from 42.3–46.8 wt% to 7 wt%), but can also result in complete lignin conversion by providing an excess amount of active hydrogen. The reaction at 350 °C for a short duration of 60 min led to the complete decomposition of alkali lignin and afforded a high yield of aromatic derivatives (36.7 wt%), while at the same time, suppressing ethanol consumption (11.8 wt%) and the formation of ethanol-derived liquid products. The alkylation of lignin-derived phenolic intermediates at the expense of the solvent is a time-dependent reaction, instead of the primary stabilization reaction. Molecular dynamics simulations using dilignol molecules revealed that the ethanol–formic acid mixture reduced the activation and thermal energies required for the dissociation of C–C and C–O bonds in the lignin structure.


 Please wait while we load your content...
Please wait while we load your content...