Efficient ionic liquid-based platform for multi-enzymatic conversion of carbon dioxide to methanol†
Abstract
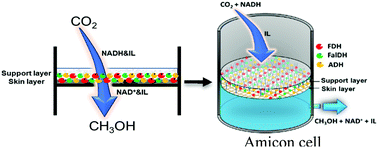
Low yields commonly obtained during enzymatic conversion of CO2 to methanol are attributed to low CO2 solubility in water. In this study, four selected ionic liquids with high CO2 solubility were separately added to the multi-enzyme reaction mixture and the yields were compared to the pure aqueous system (control). In an aqueous 20% [CH][Glu] system, yield increased ca. 3.5-fold compared to the control (ca. 5-fold if NADH regeneration was incorporated). Molecular dynamics simulation revealed that CO2 remains for longer in a productive conformation in the enzyme in the presence of [CH][Glu], which explains the marked increase of yield that was also confirmed by isothermal titration calorimetry – lower energy (ΔG) binding of CO2 to FDH. The results suggest that the accessibility of CO2 to the enzyme active site depends on the absence/presence and nature of the ionic liquid, and that the enzyme conformation determines CO2 retention and hence final conversion.


 Please wait while we load your content...
Please wait while we load your content...