Parallel anti-sense two-step cascade for alcohol amination leading to ω-amino fatty acids and α,ω-diamines†
Abstract
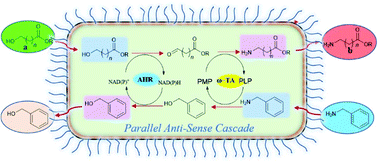
Running two two-step cascades in parallel anti-sense to transform an alcohol to an amine allowed the conversion of ω-hydroxy fatty acids (ω-HFAs) and α,ω-diols to the corresponding ω-amino fatty acids (ω-AmFAs) and α,ω-diamines, respectively. The network required only two enzymes namely an aldehyde reductase (AHR) and a transaminase (TA). Benzylamine served on the one hand as amine donor and on the other hand after deamination to benzaldehyde also as oxidant. All ω-HFAs tested were efficiently transformed to their corresponding ω-AmFAs using purified enzymes as well as a whole-cell system, separately expressing both the enzymes, with conversions ranging from 80–95%. Additionally, a single-cell co-expressing all enzymes successfully produced the ω-AmFAs as well as the α,ω-diamines with >90% yield. This system was extended by employing a lactonase, enabling the transformation of ε-caprolactone to its corresponding ω-AmFA with >80% conversion.


 Please wait while we load your content...
Please wait while we load your content...