Synthesis of α-aminonitriles using aliphatic nitriles, α-amino acids, and hexacyanoferrate as universally applicable non-toxic cyanide sources†
Abstract
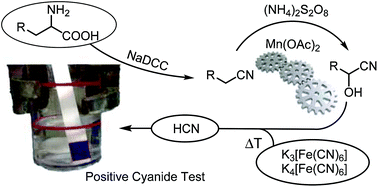
In cyanation reactions, the cyanide source is often directly added to the reaction mixture, which restricts the choice of conditions. The spatial separation of cyanide release and consumption offers higher flexibility instead. Such a setting was used for the cyanation of iminium ions with a variety of different easy-to-handle HCN sources such as hexacyanoferrate, acetonitrile or α-amino acids. The latter substrates were first converted to their corresponding nitriles through oxidative decarboxylation. While glycine directly furnishes HCN in the oxidation step, the aliphatic nitriles derived from α-substituted amino acids can be further converted into the corresponding cyanohydrins in an oxidative C–H functionalization. Mn(OAc)2 was found to catalyze the efficient release of HCN from these cyanohydrins or from acetone cyanohydrin under acidic conditions and, in combination with the two previous transformations, permits the use of protein biomass as a non-toxic source of HCN.


 Please wait while we load your content...
Please wait while we load your content...