Complex coacervation of natural sophorolipid bolaamphiphile micelles with cationic polyelectrolytes†
Abstract
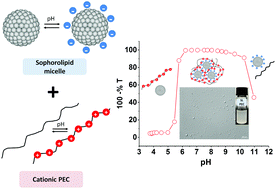
Complex coacervation of polyelectrolytes with surfactant micelles is a promising system for a wide range of applications. However, the development of “green coacervates” from bio-based surfactants and biopolymers has not yet been explored. Herein, complex coacervation of micelles from a bolaform sophorolipid biosurfactant with oppositely charged cationic polyelectrolytes (i.e., chitosan oligosaccharide lactate, poly (L-lysine) and poly(allylamine)) was investigated. Turbidity titration, light and scanning electron microscopy (SEM), dynamic light scattering (DLS), cryogenic transmission electron microscopy (cryo-TEM) and Small Angle X-ray Scattering (SAXS) were used to monitor the evolution of complex structures as a function of pH and polyelectrolyte concentration. Phase boundaries of the biosurfactant–polyelectrolyte systems were obtained and these revealed the feasibility of coacervation in water over a broad range of pH, from 5 to 9. The state of complexation was found to depend primarily on pH and concentration and the nature of the polyelectrolyte. Light microscopy and SEM demonstrated the associative macrophase separation, and cryo-TEM highlighted the influence of the desolvation level on the coacervate arrangement where two main structures were formed as a function of the coacervation stage, namely spherical particles and aggregates. The SAXS data demonstrated that the sophorolipid micelles maintained their structure integrity following their binding to the cationic polyelectrolyte.


 Please wait while we load your content...
Please wait while we load your content...