Rationally designing hydrophobic UiO-66 support for the enhanced enzymatic performance of immobilized lipase
Abstract
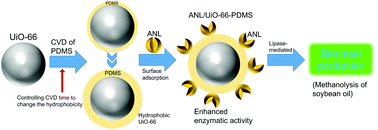
The immobilization of Aspergillus niger lipase (ANL) on UiO-66 (a typical MOF), the effect of support hydrophobicity on lipase's immobilization as well as the enzymatic performance for biodiesel preparation was explored in this enzyme/MOF system. First, the hydrophobic UiO-66 was obtained by polydimethylsiloxane (PDMS) coating, and then used as the immobilization carrier for physically adsorbing ANL through hydrophobic interactions. It was found that the properties of immobilized ANL were remarkably enhanced through hydrophobic modification of UiO-66. ANL immobilized on hydrophobic UiO-66 presented much higher enzymatic activity and activity recovery compared with those of ANL on pristine UiO-66. In the catalysis of lipase-mediated biodiesel production, ANL immobilized on hydrophobic UiO-66-PDMS-6 h performed at a faster reaction rate and gave a much higher yield than its counterpart. A biodiesel yield of 88% was afforded by ANL/UiO-66-PDMS-6 h at 24 h, and the immobilized lipase demonstrated a rather good reusability, with 83.0% activity remaining after ten successive operations.


 Please wait while we load your content...
Please wait while we load your content...