A borrowing hydrogen methodology: palladium-catalyzed dehydrative N-benzylation of 2-aminopyridines in water†
Abstract
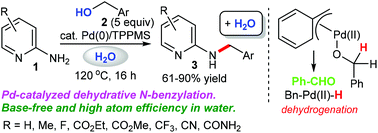
We demonstrate a greener borrowing hydrogen methodology using the π-benzylpalladium system, which offers an efficient and environmentally friendly dehydrative N-monobenzylation of 2-aminopyridines with benzylic alcohols in the absence of base. The crossover experiment using benzyl-α,α-d2 alcohol and 3-methylbenzyl alcohol afforded H/D scrambled products, suggesting that the dehydrative N-benzylation in our catalytic system involves a borrowing hydrogen pathway. KIE experiments show that C–H bond cleavage at the benzylic position of benzyl alcohol is involved in the rate-determining step (KIE = 2.9). This simple base-free protocol can be achieved under mild conditions in an atom-economic process, affording the desired products in moderate to excellent yields.


 Please wait while we load your content...
Please wait while we load your content...