Co-catalysis over a bi-functional ligand-based Pd-catalyst for tandem bis-alkoxycarbonylation of terminal alkynes†
Abstract
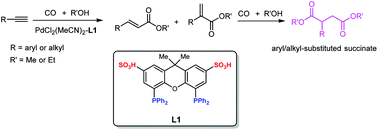
A bi-functional ligand (L1) containing a diphosphino fragment and sulfonic acid group (–SO3H) enabled PdCl2(MeCN)2 to efficiently catalyze the tandem bis-alkoxycarbonylation of terminal alkynes to produce aryl-/alkyl-substituted succinate (α,ω-diesters). It was found that the –SO3H incorporated in L1 indispensably assisted the Pd-catalyst in accomplishing this tandem reaction via intramolecular synergic effects. Co-catalysis over the L1-based Pd-catalyst was not due to the physical mixture of Xantphos and MeSO3H. In situ FTIR analysis verified that the formation and stability of Pd–H active species were facilitated by the presence of L1. The formation of stabilized diacylpalladium intermediate (F) was the critical driving force for the second-step methoxycarbonylation. DFT calculation was carried out to optimize the geometric structure of F, which indicated that the developed intramolecular O⋯H hydrogen bonds were an important structural feature to stabilize F. In addition, the L1-based Pd-catalyst could be recycled successfully for at least 3 runs in the ionic liquid [Bmim]NTf2 without obvious activity loss and detectable metal leaching.


 Please wait while we load your content...
Please wait while we load your content...