One-pot catalytic selective synthesis of 1,4-butanediol from 1,4-anhydroerythritol and hydrogen†
Abstract
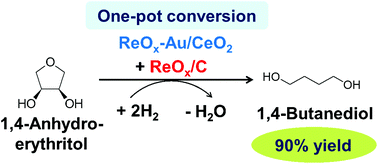
A physical mixture of ReOx–Au/CeO2 and carbon-supported rhenium catalysts effectively converted 1,4-anhydroerythritol to 1,4-butanediol with H2 as a reductant. The combination of these two catalysts in a one-pot reaction dramatically increased the selectivity of 1,4-butanediol as well as the conversion of 1,4-anhydroerythritol. The yield of 1,4-butanediol reached ∼90%, which is the highest yield from erythritol and 1,4-anhydroerythritol so far, furthermore, at a relatively low reaction temperature of 413 K. This reaction involves the ReOx–Au/CeO2-catalyzed deoxydehydration of 1,4-anhydroerythritol to 2,5-dihydrofuran and ReOx/C-catalyzed successive isomerization, hydration and reduction reactions of 2,5-dihydrofuran.


 Please wait while we load your content...
Please wait while we load your content...