Iridium-catalyzed efficient reduction of ketones in water with formic acid as a hydride donor at low catalyst loading†
Abstract
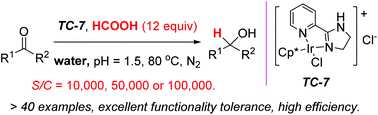
A highly efficient and chemoselective transfer hydrogenation of ketones in water has been successfully achieved with our newly developed catalyst. Simple ketones, as well as α- or β-functionalized ketones, are readily reduced. Formic acid is used as a traceless hydride source. At very low catalyst loading (S/C = 10 000 in most cases; S/C = 50 000 or 100 000 in some cases), the iridium catalyst is impressively efficient at reducing ketones in good to excellent yields. The TOF value can be as high as up to 26 000 mol mol−1 h−1. A variety of functional groups are well tolerated, for example, heteroaryl, aryloxy, alkyloxy, halogen, cyano, nitro, ester, especially acidic methylene, phenol and carboxylic acid groups.


 Please wait while we load your content...
Please wait while we load your content...