Catalytic conversion of 5-hydroxymethylfurfural to some value-added derivatives
Abstract
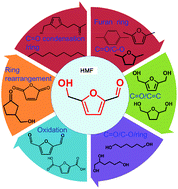
5-Hydroxymethylfurfural (HMF) is a platform chemical derived from C6 sugars, which can be transformed into various important chemicals and fuels because of the presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–O and furan ring functional groups. In this review, the selective tailoring of these groups in HMF to form 2,5-dimethylfuran, 2,5-dihydromethylfuran, 2,5-dihydromethyltetrahydrofuran, 5-ethoxymethylfurfural, 1,6-hexanediol, long-chain alkanes, 3-(hydroxy-methyl)cyclopentanone, p-xylene, 2,5-diformylfuran, 2,5-furandicarboxylic acid and maleic anhydride will be described to gain more insight into the transformation of HMF under various conditions. The focus of this review is on the mechanisms of the catalytic processes and potential design strategies for future catalysts. The activation of the functional groups and the key challenges involved in the precise design of bifunctional catalysts are highlighted. Some examples of “one-pot” transformations of fructose into various chemicals using the HMF platform are also presented.
O, C–O and furan ring functional groups. In this review, the selective tailoring of these groups in HMF to form 2,5-dimethylfuran, 2,5-dihydromethylfuran, 2,5-dihydromethyltetrahydrofuran, 5-ethoxymethylfurfural, 1,6-hexanediol, long-chain alkanes, 3-(hydroxy-methyl)cyclopentanone, p-xylene, 2,5-diformylfuran, 2,5-furandicarboxylic acid and maleic anhydride will be described to gain more insight into the transformation of HMF under various conditions. The focus of this review is on the mechanisms of the catalytic processes and potential design strategies for future catalysts. The activation of the functional groups and the key challenges involved in the precise design of bifunctional catalysts are highlighted. Some examples of “one-pot” transformations of fructose into various chemicals using the HMF platform are also presented.


 Please wait while we load your content...
Please wait while we load your content...