Conversion of glucose into 5-hydroxymethylfurfural catalyzed by acid–base bifunctional heteropolyacid-based ionic hybrids†
Abstract
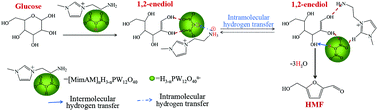
The design of stable acid–base bifunctional heterogeneous catalysts has become crucial for efficient catalytic conversion of renewable biomass to high value-added chemicals. Heteropolyacids (HPAs) are of great interest due to their unique tunable structures and suitable acidity. In this study, a series of acid–base bifunctional ionic hybrid catalysts [MimAM]nH3−nPW12O40 (n = 1–3) were synthesized by the ion exchange method using an amino-functionalized imidazolium ionic liquid and H3PW12O40 as precursors. The introduction of the ionic liquid resulted in a varied Lewis–Brønsted acidity for [MimAM]nH3−nPW12O40. The synergistic effect of dual acidic properties endowed [MimAM]H2PW12O40 with more efficiency for glucose dehydration in THF/H2O–NaCl with 53.9% HMF yield. A unique sequential hydrogen transfer mechanism is proposed to understand the efficient heterogeneous catalysis. Among them, the absence of fructose and levoglucosenone in glucose dehydration may come from the formation of stabilized intermediates 1,2-enediol and anhydrosugars due to the existence of an effective hydrogen bond between O–H and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) W on the heteropoly anion; the reversible process between glucose dehydration and anhydrosugar hydration is responsible for the absence of HMF during the early stage of reaction. This work provides a deep understanding of the HPA-catalyzed conversion of glucose to HMF.
W on the heteropoly anion; the reversible process between glucose dehydration and anhydrosugar hydration is responsible for the absence of HMF during the early stage of reaction. This work provides a deep understanding of the HPA-catalyzed conversion of glucose to HMF.


 Please wait while we load your content...
Please wait while we load your content...